Today the FDA recalled Synex II vertebral implants. The implants were recalled because they pose an imminent health hazard. Six adverse event reports have been filed to date which demonstrated moderate to severe loss of vertebral body replacement height. Other potential complications included neural injury, increased pain and need for reoperation/revision surgery. Synthes had a previous recall in Feb 2009.
In a press release Synthes advised: “that surgeons and hospitals in possession of the subject devices must stop implanting them immediately.”
Vertebral implants are utilized in lumbar spine surgery and were thought to have a potential advantage over conventional fusion techniques.
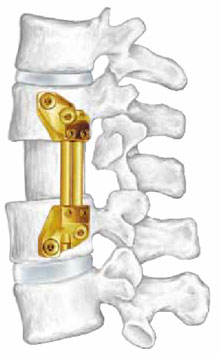
Surgery is a one way door: once undertaken one can only move forward. Complications are part of the risks of surgery. Removal of the disc, which functions as a shock absorber, can result in pressure overload in adjacent levels thereby advancing the degenerative casade.
A non-surgical option for the treatment of lumbar disc disease is the use of your own stem cells. Utilizing the Regenexx procedure, patients have had reduction in their back and leg pain.